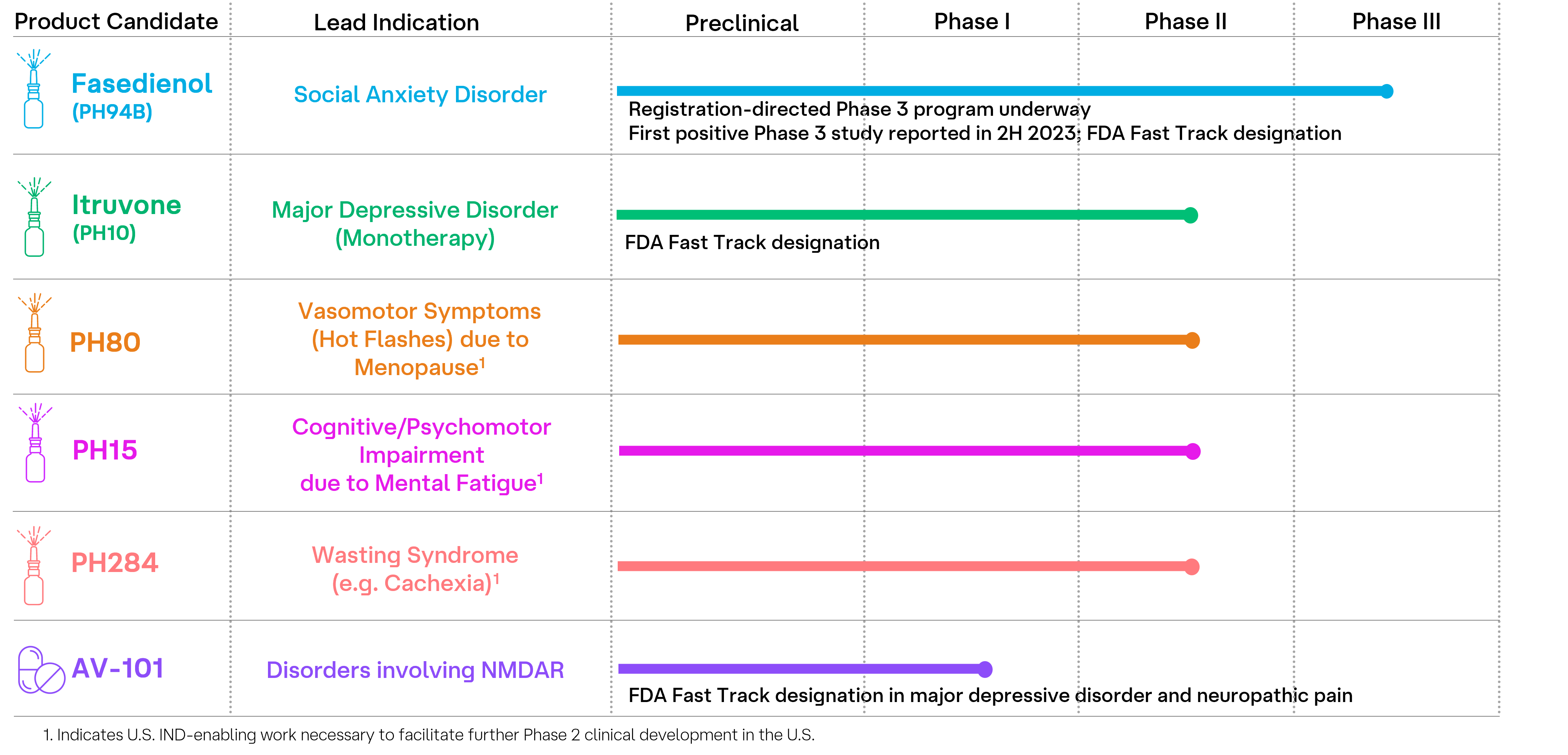
Neuroscience Pipeline Overview
Our robust neuroscience pipeline includes five clinical-stage investigational agents belonging to a new class of drugs known as pherines and one oral antagonist of the glycine site of the N-methyl-D-aspartate receptor (NMDAR). Our experienced team and extensive knowledge of these innovative drug candidates drive our clinical efforts as we work to deliver groundbreaking therapies for individuals affected by psychiatric and neurological disorders.